某同学取一定量稀盐酸于烧杯中,向其中加入8g氧化铁,二者恰好完全反应,若将氧化铁换成氢氧化钠固体,要想与上述稀盐酸恰好完全反应,需要加入氢氧化钠的质量为( )
- A.4 g
- B.8 g
- C.12 g
- D.无法计算
答案
正确答案:C
根据题意,要想计算加入氢氧化钠的质量,需知道稀盐酸中HCl的质量,而HCl的质量可根据与之反应的氧化铁的质量计算。利用分步计算或关系式法计算均可。
(1)分步计算
①设稀盐酸中HCl的质量为x
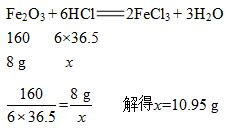
②设需加入的氢氧化钠的质量为y
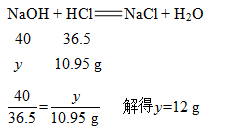
(2)关系式法
根据发生的反应Fe2O3+6HCl![]() 2FeCl3 + 3H2O和NaOH +HCl
2FeCl3 + 3H2O和NaOH +HCl![]() NaCl+H2O,确定出相关物质的关系为Fe2O3~6HCl~6NaOH,然后直接确定已知量(Fe2O3的质量)和未知量(NaOH的质量)的关系,即Fe2O3~6NaOH。
NaCl+H2O,确定出相关物质的关系为Fe2O3~6HCl~6NaOH,然后直接确定已知量(Fe2O3的质量)和未知量(NaOH的质量)的关系,即Fe2O3~6NaOH。
设需加入的氢氧化钠的质量为z
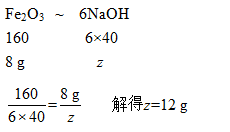
根据上述计算可知,需加入12 g的氢氧化钠,故选C。
略







